The exception proves the rule. A new ferrocene derivative with 20 electrons completely defies the best-known rule of stability in chemistry, opening up unprecedented avenues for the design of catalysts, electronic materials and advanced redox compounds.
For generations, chemistry students have learned that the most stable metal compounds follow a well-defined pattern: the rule of 18 electrons. This rule, found in textbooks and university lectures, states that metal complexes tend to stabilise when the central atom reaches 18 valence electrons. But recent research could force a rethink of this seemingly immutable principle.
An international group of scientists has achieved what was previously considered virtually impossible: synthesising a stable and functional ferrocene derivative with 20 electrons, openly challenging one of the pillars of organometallic chemistry. The study, published in Nature Communications and led by the team at the Okinawa Institute of Science and Technology (OIST), proposes a new scenario for understanding the stability and reactivity of certain metal compounds. Far from being a technical detail, this finding could have a direct impact on the way new materials, catalysts and components for electronic devices are designed.ScienceYou won’t believe it: biology textbooks need to be rewritten because neurons are not shaped as we thoughtEugenio M. Fernández Aguilar
Ferrocene and the rule that seemed untouchable
Ferrocene is an iconic compound in chemistry, known since the 1950s for its peculiar ‘sandwich’ structure, where an iron atom is sandwiched between two cyclopentadiene rings. Its discovery was so revolutionary that it earned its discoverers the Nobel Prize in Chemistry in 1973. Beyond its academic interest, ferrocene has found applications in batteries, sensors, medicines and advanced materials.
Its stability has always been explained by its electronic configuration: 18 valence electrons distributed symmetrically and evenly. This quantity allows for a diamagnetic state and a very favourable energy distribution. In fact, attempts to add more electrons to this type of compound, especially through the coordination of additional ligands, were considered unfeasible or, at best, short-lived.
Therefore, the formation of a ferrocene compound with 20 electrons—stable not only under controlled conditions but also in solution at room temperature—represents a paradigm shift. This possibility had never before been observed in a stable form in diamagnetic iron complexes with a d6 configuration, which are typical of ferrocene derivatives.
The rule broken. On the right, the type of bond that had never been achieved: a nitrogen base coordinated to iron in ferrocene. Source: Nature Communications
A century of stability: the origin of the 18-electron rule
In 1921, American chemist Irving Langmuir proposed the 18-electron rule as an extension of Lewis’s octet rule. He observed that transition metal complexes tend to be more stable when the central atom is surrounded by 18 valence electrons, thus completing its s, p and d orbitals. This configuration resembles that of noble gases, known for their chemical inertness. Langmuir applied this idea to compounds such as Ni(CO)₄, Fe(CO)₅ and Mo(CO)₆, establishing a pattern that became a fundamental principle of organometallic chemistry.
Throughout the 20th century, the 18-electron rule became established as an essential tool for predicting the stability of metal complexes, especially in organometallic chemistry. Its usefulness was backed by Nobel Prize-winning discoveries, such as the 1973 award to Ernst Otto Fischer and Geoffrey Wilkinson for their work on ‘sandwich’ compounds such as ferrocene. These advances demonstrated how the 18-electron rule could guide the synthesis and understanding of new materials with unique properties.
The molecular design that challenged the norm
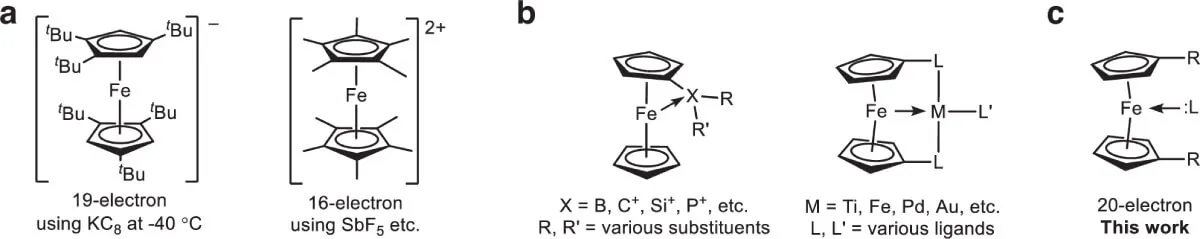
The key to the discovery lies in the use of a ligand system specifically designed to induce intramolecular coordination of nitrogen to the iron atom. The researchers used a variant of ferrocene that incorporates a modified pyridine unit, thus creating an internal interaction that allows a pair of electrons to be added to the complex without compromising its structure.
According to the original article, ‘we report the formation of ferrocene derivatives with 20 electrons through reversible coordination of nitrogen to 18-electron analogues.’ This coordination, although in principle contrary to classical predictions, was achieved thanks to a precise design of the molecular environment, which favours interaction without altering the typical arrangement of the Cp (cyclopentadienyl) rings.
Under laboratory conditions, the resulting compound not only showed a clear structure by X-ray diffraction, but was also stable for more than a year under a nitrogen atmosphere. In the solid state, it behaves like an 18-electron diamagnetic complex, but in solution a dynamic equilibrium is established between two species: one without Fe–N coordination (18e) and the other with active coordination (20e).
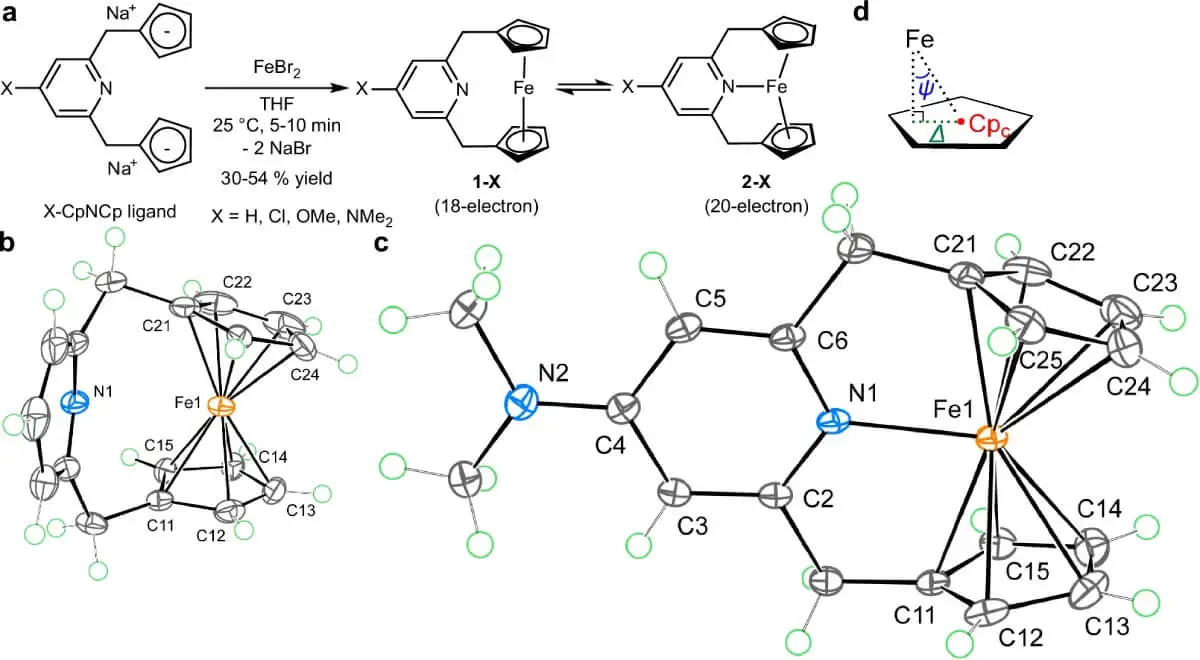
The impossible molecule (c). Crystallographic view of the new iron compound with 20 electrons, showing the unprecedented Fe–N bond. Source: Nature Communications
Unexpected redox behaviour
One of the most surprising aspects of the new compound is its ability to participate in redox reactions in a controlled manner. Traditional ferrocenes can be oxidised to a 17-electron state with relative ease, but reaching higher states, such as 16 or 14 electrons, requires extreme conditions.
This new derivative, on the other hand, allows sequential oxidation from iron(II) to iron(IV) under mild conditions and with proven reversibility. In the words of the article: ‘these 20-electron ferrocene derivatives exhibit reversible Fe(II)/Fe(III)/Fe(IV) redox chemistry under previously unattainable mild conditions.’
This finding is no small matter. It means that these types of compounds can act as mediators in reactions requiring controlled electron transfer, opening the door to new applications in catalysis, energy storage and molecular electronics. This is a particularly significant advance for the development of materials with adjustable redox properties.
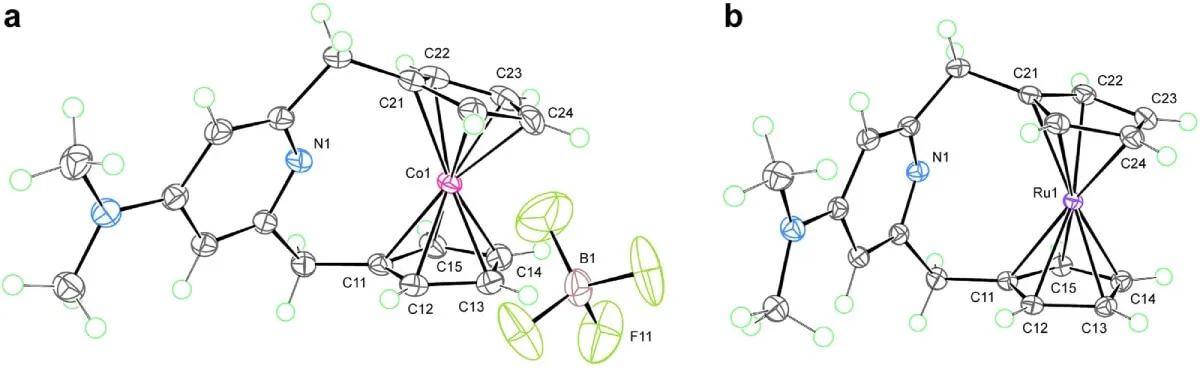
Other metals cannot achieve this. Attempts to form the same type of bond with cobalt (left) and ruthenium (right) were unsuccessful, highlighting the rarity of the iron compound. Source: Nature Communications
Profound changes in chemical bonds
At the theoretical level, the researchers performed density functional theory (DFT) and quantum chemical topology calculations to understand why this coordination was possible. What they found was that the formation of the Fe–N bond changes the way iron interacts with the Cp rings, making the interactions less covalent and more ionic.
This change has a direct effect on the molecular orbitals: occupied anti-bonding orbitals appear that weaken the Fe–Cp bond, but at the same time facilitate the stabilisation of the new Fe–N bond. According to the study, ‘the coordination of N1 to Fe1 leads to a significant reorganisation of the zero-current surfaces’ and results in a ‘notable increase in charge transfer from the metal to the ligand’.
The ability to observe this type of electronic reorganisation with such detailed precision represents a remarkable technical advance and allows us to envisage how new compounds could be designed that break other structural rules.
Is this an exception or the beginning of something bigger?
Although this discovery marks a milestone, the authors of the study are cautious. Not all 18-electron complexes could be modified in this way. In fact, similar attempts with cobaltocene or metals such as ruthenium did not produce the same results.
As they explain, the feasibility of this additional coordination seems to depend on the balance between the strength of the existing bonds and the ability of the additional ligand to stabilise new electronic states.
This suggests that this type of transformation would be more common in neutral complexes of first-row metals (such as iron), which have weaker metal-ligand interactions. In these cases, the possibility of introducing accessible anti-bonding orbitals would facilitate the formation of stable 20-electron compounds.
Although it is still too early to talk about a revolution, the study does suggest that the 18-electron rule may not be as rigid as previously thought, at least under certain conditions and with appropriate molecular design.

